The Synthesis of Azadirachtin: A Potent Insect Antifeedant
Steven V Ley, Antonio Abad-Somovilla, James C Anderson, Carles Ayats, Rolf Bänteli, Edith Beckmann, Alistair Boyer, Maria G Brasca, Abigail Brice, Howard B Broughton, Brenda J Burke, Ed Cleator, Donald Craig, Alastair A Denholm , Ross M Denton, Thomas Durand-Reville, Luca B Gobbi, Michael Göbel, Brian Lawrence-Gray, Robert B Grossmann, Claire E Gutteridge, Norbert Hahn, Sarah L Harding, David C Jennens, Lynn Jennens, Peter J Lovell, Helen J Lovell, Mary L de la Puente, Hartmuth C Kolb, Win-Jan Koot, Sarah L Maslen, Catherine F McCusker, Amos Mattes, Andrew R Pape, Andrea Pinto , Dinos Santafianos, James S Scott, Stephen C Smith, Andrew Q Somers, Christopher D Spilling, Frank Stelzer, Peter L Toogood, Richard M Turner, Gemma E Veitch, Anthony Wood, Cornelia Zumbrunn
Chem. Eur. J.
2008
14
34
10683
10704
VIP paper
Highlighted in "Classics in Total Synthesis III"
[Link]This work has been cited 49 times
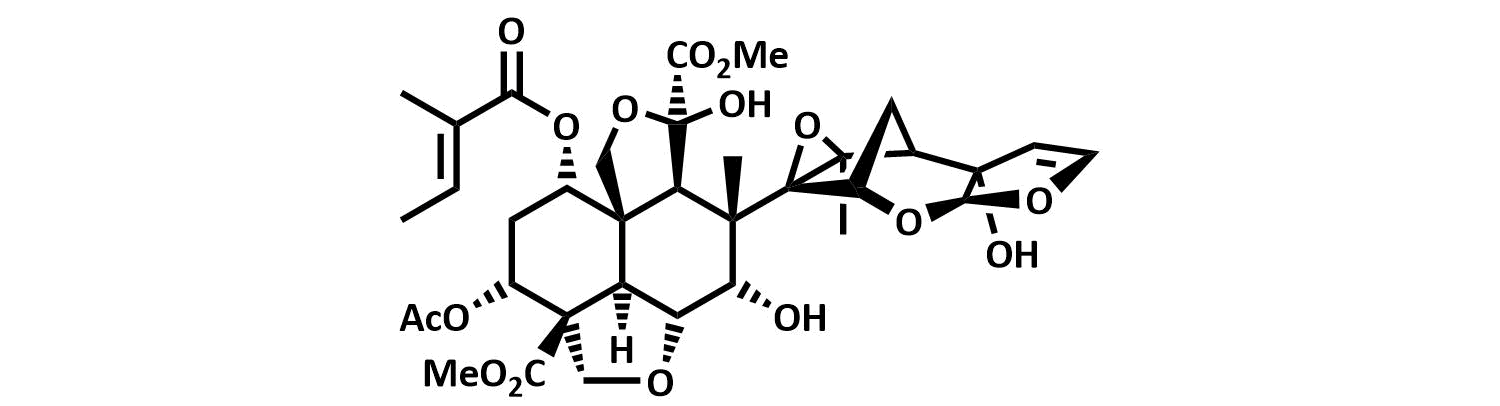
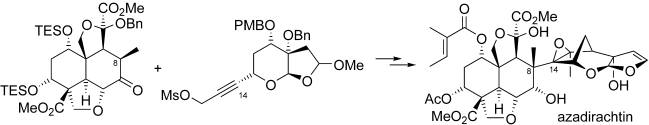
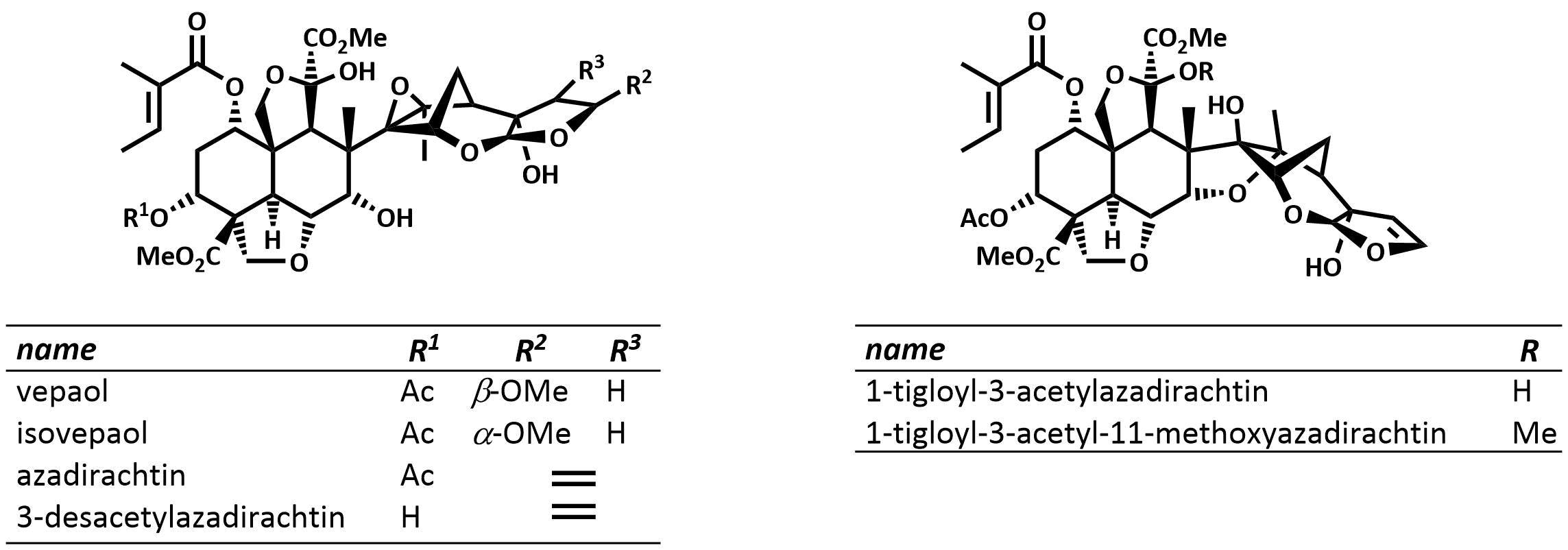
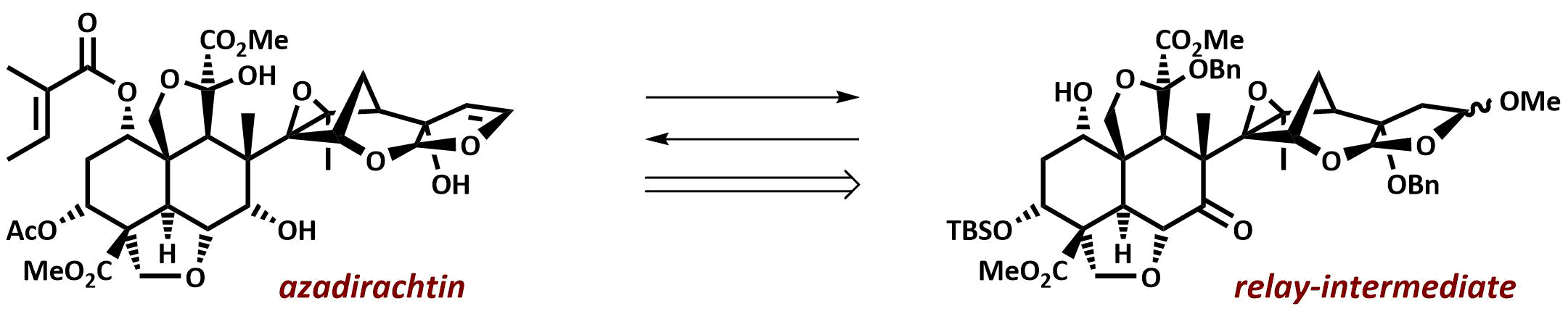
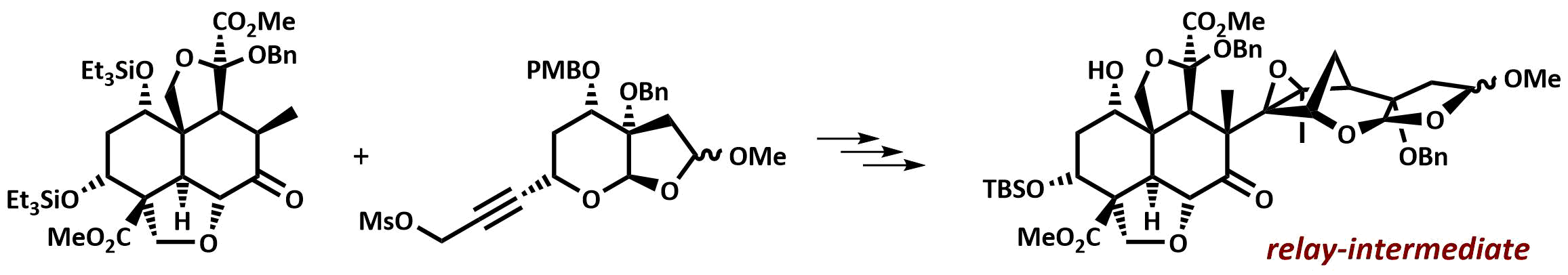
The final chapter! We describe in full the first synthesis of the potent insect antifeedant azadirachtin through a highly convergent approach. An O-alkylation reaction is used to unite decalin ketone and propargylic mesylate fragments, after which a Claisen rearrangement con structs the central C8-C14 bond in a stereoselective fashion. The allene which results from this sequence then enables a second critical carbon-carbon bond forming event whereby th e [3.2.1] bicyclic system, present in the natural product, is generated via a 5-exo-radical cyclisation process. Finally, using knowledge gained through our early studies into the reactivity of the natural product, a series of carefully designed steps completes the synthesis of this challenging molecule.

















![Abstract image for Rhodium-Catalysed Domino Enantioselective Synthesis of Bicyclo[2.2.2]lactones](publications/10.1002_anie.201101773.png)












![Abstract image for Synthesis of Substituted Indazoles via [3 + 2] Cycloaddition of Benzyne and Diazo Compounds](publications/10.15227_orgsyn.087.0095.png)